DYANAVEL® XR delivers
Eligible patients may PAY $25
Learn more about Savings & SupportDYANAVEL XR (amphetamine) tablet is proven to be bioequivalent to DYANAVEL XR oral suspension.2 See PK Data.
DYANAVEL XR (amphetamine) Oral Solution Pediatric Study
Continuous symptom control with no return to baseline at 13 hours in an optimized-dose,
double-blind phase 3 study1
Improvement in Attention and Behavior With
DYANAVEL® XR (amphetamine) Oral Suspension vs Placebo1

Primary endpoint: Significant improvement in SKAMP-combined score at 4 hours postdose vs placebo (P<0.0001)*1
Study Design: This dose-optimized, randomized, double-blind, placebo-controlled laboratory classroom study included 99 children aged 6 to 12 years who met DSM-IV-TR criteria for ADHD. In the 5 week open-label dose-optimization period, patients received a starting dose of 2.5 or 5mg of DYANAVEL XR taken once daily in the morning. The dose was titrated by 2.5mg to 10mg increments every 4 to 7 days until an optimal dose or the maximum daily dose of 20mg/day was reached. Patients who achieved an optimal dose during the dose optimization period entered the double blind portion of the study where they were randomized to either DYANAVEL XR at their optimal dose or placebo once daily for one week. Efficacy was assessed on the final day of the double-blind phase, by teachers and raters using the SKAMP rating scale. The primary endpoint was the change from predose in the model-adjusted average of SKAMP-combined score at 4 hours postdose. Secondary endpoints looked at change from predose SKAMP-combined scores at 1, 2, 6, 8, 10, 12 and 13 hours postdose.
*The SKAMP (Swanson, Kotkin, Agler, M-Flynn, and Pelham) rating scale, often used in clinical trials, is a validated rating instrument, used by trained raters to specifically measure the observed classroom manifestations of ADHD. The items of the SKAMP are specific for place (the classroom) and time (a typical class period). The scale's items describe typical behaviors in a classroom setting, and additional items that describe behaviors associated with ADHD in the classroom. The SKAMP method of assessment has been demonstrated to be a sensitive measure of attention and behavior within a lab classroom setting.3
LS, least squares.
PERMP Scores Were Significantly Improved With
DYANAVEL XR for up to 13 Hours Postdose vs Placebo1
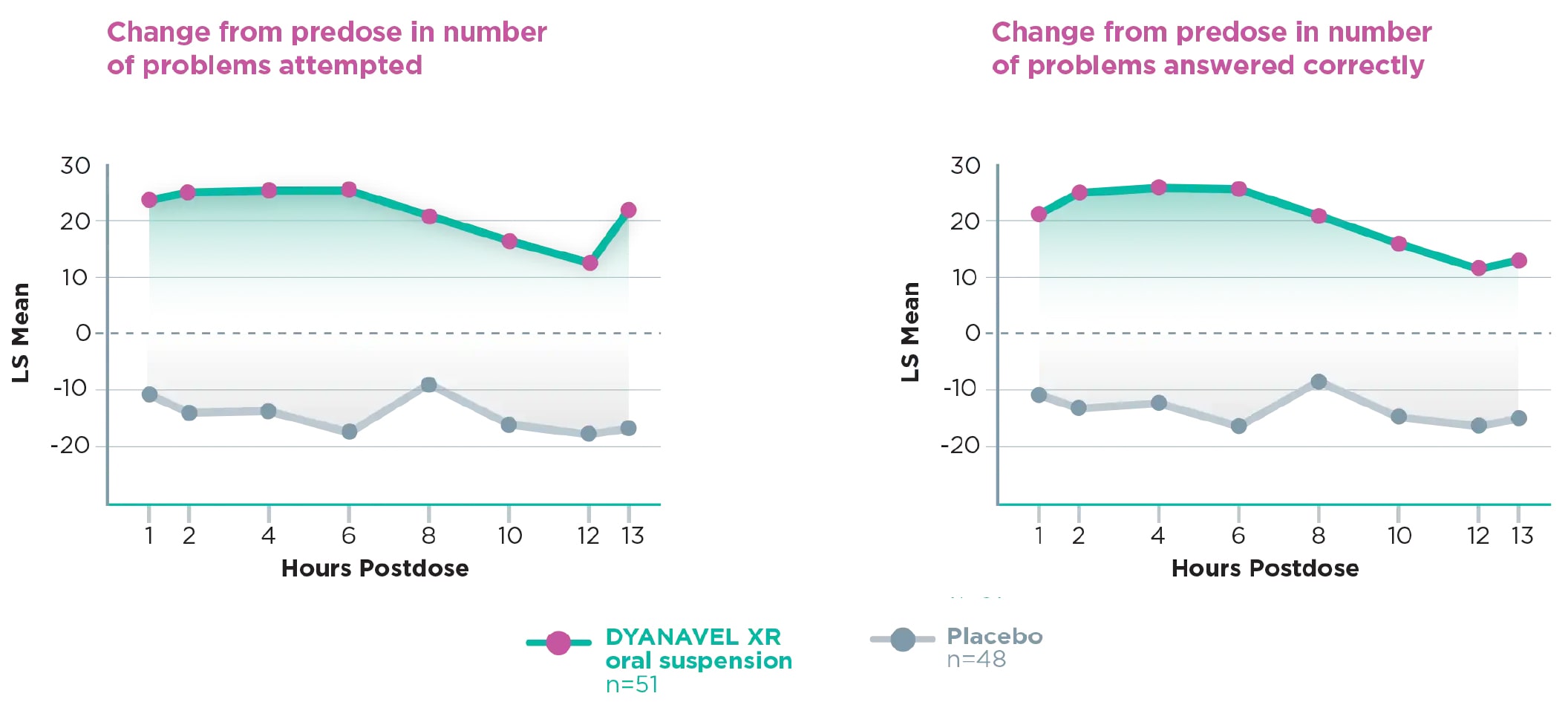
- One of the secondary endpoints was change from predose PERMP scores at 1, 2, 4, 6, 8, 10, 12, and 13 hours postdose1
- PERMP is a 10-minute written test with the number of problems attempted and the number of problems answered correctly used as measures of a patient’s performance1
- PERMP scores (problems attempted and problems answered correctly) were significantly improved with DYANAVEL XR (amphetamine) at all postdose time points compared with placebo (P<0.0001 for both)1
PERMP Test Examples
The PERMP is a validated, time-sensitive, skill-adjusted test consisting of math problems to be completed at multiple time points (administration of serial PERMPs). It is a robust, objective measure of the ability to initiate a task, self-monitor/stay on task, and complete written seatwork. The PERMP does not test for mathematical ability or the ability to learn math because the difficulty of problems is adjusted to the existing math skill level of each participant.4
Shown below are the examples of PERMP test results from an actual patient in the clinical study.
Open-Label Period
Practice Laboratory School Day (Visit 7)
DYANAVEL XR at 1 hour

Double-Blind Period
Complete Laboratory School Day (Visit 7)
Placebo at 1 hour

DYANAVEL XR (amphetamine) Tablets Adult ADHD Study
Improvement in Attention With DYANAVEL® XR (amphetamine) Tablet vs Placebo5

Primary endpoint: Mean Permanent Product Measure of Performance Total (PERMP-T)* score averaged across all postdose time points at visit 5 was significantly higher in the DYANAVEL XR group than the placebo group (P=0.0043).**
Study Details: This study employed a 5-week forced dose–titration phase. Eligible subjects aged 18 to 60 years were randomized to double-blind DYANAVEL XR tablet or matching placebo, taken orally once daily beginning the day after the baseline visit. Subjects were titrated from a 5mg starting dose up by 5-mg increments each week until a final dose of 20mg for 14 ± 3 days prior to visit 5. Subjects who could not tolerate the study drug were discontinued. At Visit 5, efficacy assessments included the administration of serial PERMPs predose and at 0.5, 1, 2, 4, 8, 10, 12, 13, and 14 hours postdose. Safety and tolerability were assessed at each study visit, including direct questioning about sleep, appetite, mood, and psychotic adverse events.5
*The PERMP is a validated, time-sensitive, skill-adjusted test consisting of math problems to be completed at multiple time points (administration of serial PERMPs). It is a robust, objective measure of the ability to initiate a task, self-monitor/stay on task, and complete written seatwork. The PERMP does not test for mathematical ability or the ability to learn math because the difficulty of problems is adjusted to the existing math skill level of each participant.4
**Mean PERMP-T score across all post dose time points at Visit 5 (last visit) was 302.8 for DYANAVEL XR tablet and 279.6 for placebo.5
Safety data for DYANAVEL XR
References: 1. Childress AC, Wigal SB, Brams MN, et al. Efficacy and safety of amphetamine extended-release oral suspension in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psycholpharmacol. 2018;28(5):306-313. 2. Pardo A, Kando JC, King TR, Rafla E, Herman BK. Single-dose pharmacokinetics of amphetamine extended-release tablets compared with amphetamine extended-release oral suspension. CNS Spectr. 2020;25(6):774-781. 3. Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull. 1998;34(1):47-53. 4. Data on file. Tris Pharma, Inc. 5. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438.
